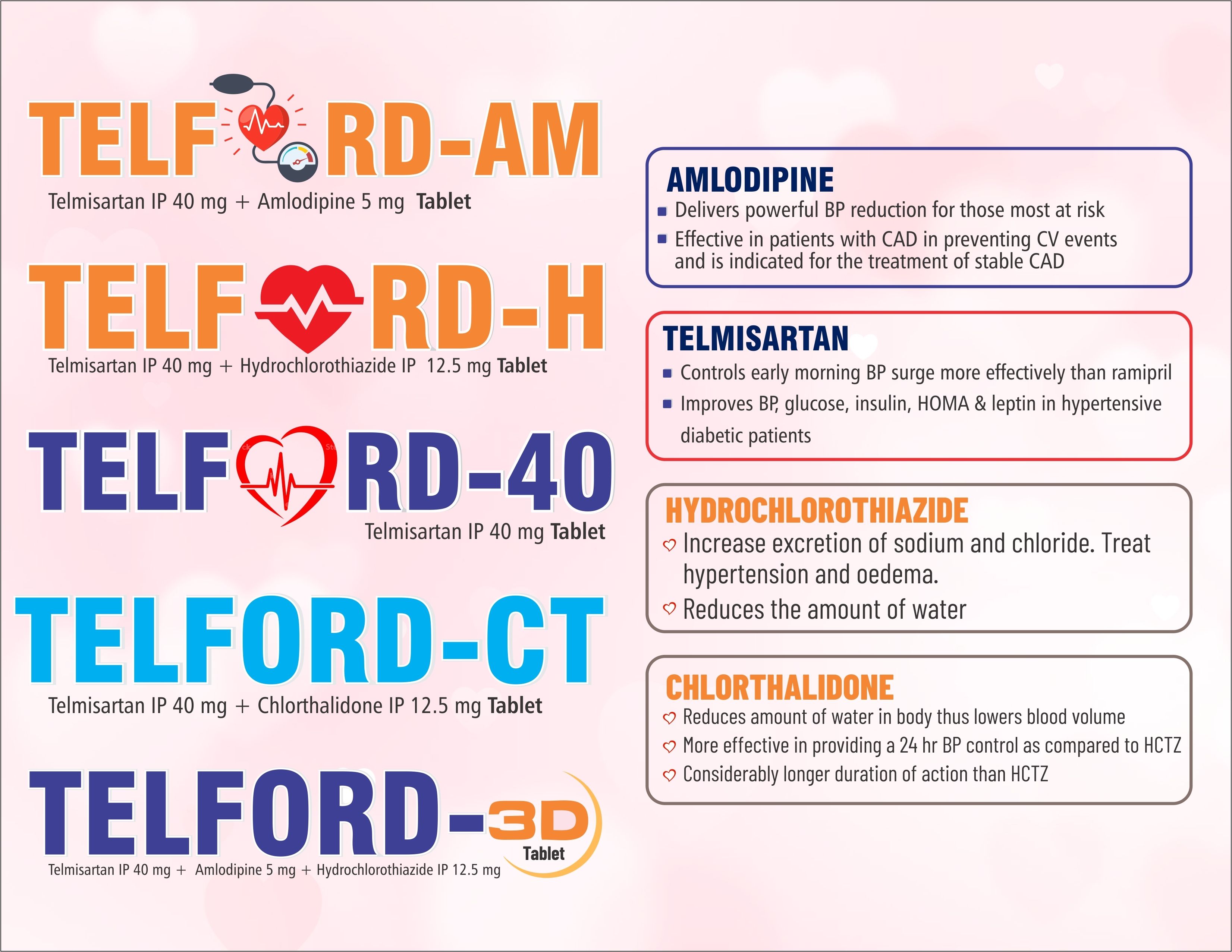
Telmisartan Chlorthalidone Tablet
16.10 INR
Product Details:
- Molecular Formula Telmisartan IP 40 mg + Chlorthalidone IP 12.5 mg
- HS Code 3004
- Type Other
- Ingredients Other
- Shelf Life 36 month Months
- Storage Instructions Dry Place
- Click to View more
X
Telmisartan Chlorthalidone Tablet Price And Quantity
- 100 Unit
- 14.00 - 16.10 INR
- 16.10 INR
Telmisartan Chlorthalidone Tablet Product Specifications
- Dry Place
- Telmisartan IP 40 mg + Chlorthalidone IP 12.5 mg
- Other
- 3004
- 36 month Months
- Other
Telmisartan Chlorthalidone Tablet Trade Information
- 100000 Unit Per Month
- 1 Days
- Yes
- Free samples available with shipping and taxes paid by the buyer
- Asia
- All India
Product Description
Telford CT presents a robust solution for hypertension management with its Telmisartan Chlorthalidone Tablet formulation. Each tablet combines the potent actions of telmisartan, an angiotensin II receptor blocker, and chlorthalidone, a thiazide-like diuretic, to effectively lower blood pressure. Telmisartan acts by dilating blood vessels, facilitating smoother blood flow, while chlorthalidone promotes the elimination of excess salt and water from the body, aiding in blood pressure reduction. Telford CT offers various dosage strengths, ensuring flexibility in treatment regimens to meet individual patient needs. Manufactured under stringent quality control measures compliant with Good Manufacturing Practices (GMP), Telford CT tablets guarantee consistency, safety, and efficacy. Packaged with clear dosage instructions and accompanied by comprehensive patient information leaflets, Telford CT empowers patients with essential knowledge about the medications uses, potential side effects, precautions, and contraindications. With a commitment to improving patient outcomes and regulatory compliance, Telford CT emerges as a trusted choice in hypertension management, providing reliable support for healthier lives.FAQs of Telmisartan Chlorthalidone Tablet:
Q: What is the molecular formula of Telmisartan Chlorthalidone Tablet?
A: The molecular formula of Telmisartan Chlorthalidone Tablet is Telmisartan IP 40 mg + Chlorthalidone IP 12.5 mg.Q: What is the shelf life of Telmisartan Chlorthalidone Tablet?
A: The shelf life of Telmisartan Chlorthalidone Tablet is 36 months.Q: Under which HS Code does Telmisartan Chlorthalidone Tablet fall?
A: Telmisartan Chlorthalidone Tablet falls under HS Code 3004.Q: How should Telmisartan Chlorthalidone Tablet be stored?
A: Telmisartan Chlorthalidone Tablet should be stored in a dry place.Q: What is the type description of Telmisartan Chlorthalidone Tablet?
A: The type description for Telmisartan Chlorthalidone Tablet is listed as "Other."Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email